
Education
- Colby College, B. A. 1981
- M.I.T, Ph.D. 1987
- University of Washington, NIH Post-Doctoral Fellow, 1990-93
Professional Experience
- Genzyme Co., Boston, 1987-1990
- University of Maryland, Assistant Professor, 1993-99
- University of Maryland, Associate Professor, 1999-2003
- University of Maryland, Professor, 2003-present
- Associate Chair, Graduate Studies, 2014-2017
- Visiting Professor, University of Twente, Netherlands. 2002-2003.
Research Interests
Supramolecular Chemistry, Bioorganic Chemistry, Natural Products Chemistry
Major Recognitions and Honors
- NIH Postdoctoral Fellow, 1991-1993
- Outstanding Junior Faculty, Chemical and Life Sciences, UMCP 1997
- Camille Dreyfus Teacher-Scholar, 1998-2003
- Inaugural Chemical Society Review Lectureship, 2006
- University of Maryland Distinguished Teacher-Scholar, 2018-19
Significant Professional Service and Activities
UMCP Nanotechnology Advisory Board, 2004-present; Royal Society of Chemistry Inaugural Lecturer (2006); Organizer, 9th International Conference on Calixarene Chemistry (2007); Organizer, 9th International Conference on Calixarene Chemistry (2007); Co-Organizer, 8th International Symposium on Macrocyclic and Supramolecular Chemistry (2013)
Students Mentored
To date over 35 undergraduates have done research with Prof. Davis. He has also mentored 18 Ph. D. degree recipients and 10 M. S. degree recipients.
Supramolecular Chemistry and Molecular Self-Assembly, Hydrogels
We are making functional supramolecular structures, such as hydrogels, via self-assembly.
- Supramolecular Hydrogels from natural products have promise in drug delivery and tissue engineering. We have found
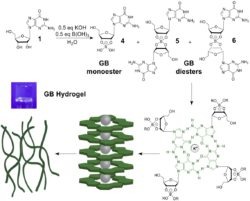 that potassium borate templates self-assembly of guanosine into a supramolecular hydrogel. This guanosine-borate (GB) hydrogel is stable in salt water and absorbs cationic dyes and antiviral drugs. We are working to determine the mechanism of formation and the range of applications for these hydrogels.
that potassium borate templates self-assembly of guanosine into a supramolecular hydrogel. This guanosine-borate (GB) hydrogel is stable in salt water and absorbs cationic dyes and antiviral drugs. We are working to determine the mechanism of formation and the range of applications for these hydrogels.
For some recent papers, see:
Magic-angle spinning NMR spectroscopy provides insight into the impact of small molecule uptake by G-quartet hydrogels GM Reddy, GM Peters et al Materials Advances 2020, 1, 2236
Drawing with iron on a gel containing a supramolecular siderophore S Xiao, PJ Paukstelis, RD Ash, PY Zavalij, JT Davis Angewandte Chemie 2019, 131, 18605-18608
Self-assembly of metallo-nucleoside hydrogels for injectable materials that promote wound closure Q Tang, TN Plank, et al ACS Applied Materials & Interfaces 2019, 11, 19743-19750
Templating and Catalyzing [2+ 2] Photocycloaddition in Solution Using a Dynamic G‐Quadruplex KB Sutyak, W Lee, PV Zavalij, O Gutierrez, JT Davis Angewandte Chemie International Edition 2018, 57, 17146-17150
Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs
GM Peters, JT Davis Chemical Society Reviews 2016, 45, 3188-3206


