
Education
- Harvard College, A. B. 1964, Magna Cum Laude
- Université de Paris-Sud, Ph. D., 1967, Orsay, France, 1967, With High Distinction
- Harvard University, Research Fellow, 1967-1971
Professional Experience
- University of Maryland, Assistant Professor, 1971-1975
- University of Maryland, Associate Professor, 1975-1979
- University of Maryland, Professor, 1979-1999
- Universtiy Distinguished Emeritus Professor, 2022-
- 1997 Co-chair, Gordon Conference on Molecular Energy Transfer
- 1997-1998 Dr. Lee’s Research Fellowship, Christ Church, Oxford University, UK
- 1997-1998 John Simon Guggenheim Memorial Fellowship
- 2004 Co-organizer, Symposium on Intermolecular Interactions and Reactions involving Ions and Open-Shell Systems, ACS Nat’l Meeting, Anaheim, CA
- 2005 Co-chair, VIII Int’l Workshop on Quantum Reactive Scattering, CA
- 2008, 2010, 2012, 2014, 2016 Chair, Telluride Winter Workshop on “New Challenges for Theory in Chemical Dynamics.”
- 2008 Member, Board of Directors, Telluride Science Research Center
- 2012 President, Board of Directors, Telluride Science Research Center
Research Interests
Theoretical study of inelastic and reactive molecular collisions, particularly those involving free radicals; the photofragmentation of small molecules; and the structure and energetics of weakly bound complexes involving open-shell species.
Major Recognitions and Honors
- NSF Predoctoral Fellow, 1964-1967
- Outstanding Young Teacher Award, D.C. Institute of Chemists, 1977
- Elected Fellow, American Physical Society, 1984.
- Alexander von Humboldt Foundation, Senior US Scientist Award, 1989
- Excellence in Teaching Award, University of Maryland, 1992
- Contribution to Science Award, Sigma Xi, 1993 Hillebrand Award, American Chemical Society, 1996
- Dr. Lee’s Visiting Research Fellowship, Christ Church, Oxford, UK, 1997
- John Simon Guggenheim Memorial Fellowship, 1997
- Kirwan Faculty Research and Scholarship Prize, University of Maryland, 1999
- Elected Fellow, American Association for Advancement of Science, 2012
- Elected Member, International Academy of Quantum Molecular Science, 2015
- Herschbach Medal, Dynamics of Molecular Collisions, 2015
Chemistry in most combustion environments involves atoms and small molecular radicals. An understanding of the chemical kinetics and relaxation pathways in conditions far from equilibrium depends critically on our ability to model accurately inelastic and reactive collisions involving radicals. Alexander’s work has provided a framework for the understanding of these processes. Utilizing state-of-the art techniques in ab initio quantum chemistry and new methods in quantum collision theory, he has pushed forward the frontier in the understanding of how electronic and nuclear motion are coupled in elementary inelastic and reactive collisions.
Alexander’s earlier work on inelastic scattering of open-shell molecules enabled the understanding of a numerous experimental studies on these systems. Similarly, his recent work on non-adiabatic effects in the paradigm F+H2, Cl+H2, and O+H2 reactions has transformed our understanding of a wealth of experimental work on these elementary reactions. The figure below illustrates how the unpaired electrons on the O atom evolve in its reaction with molecular hydrogen, 1 a reaction probed recently in crossed molecular-beam scattering studies by Minton, McKendrick and co-workers. 2
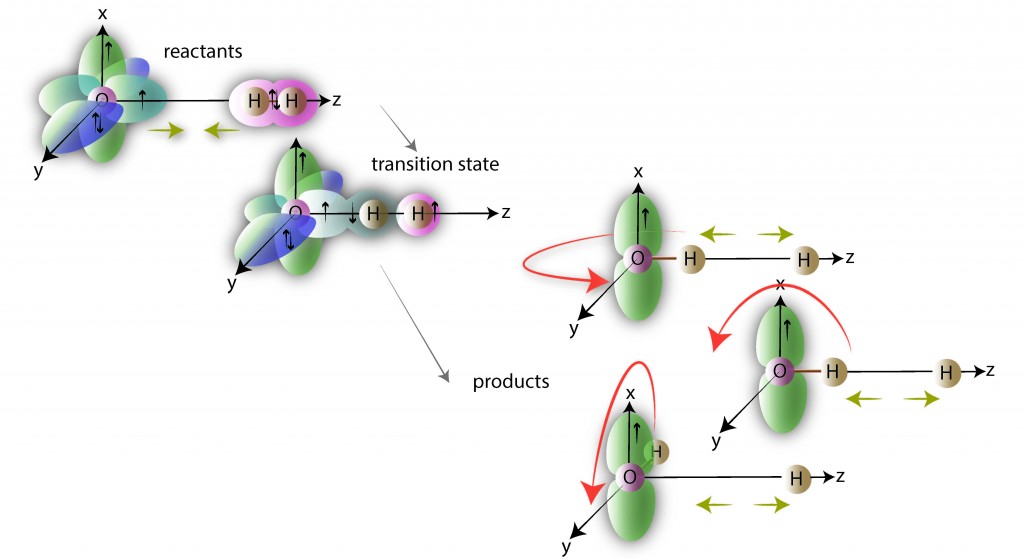
The impact of Alexander’s work on the field of chemical dynamics is witnessed by his co-authorship of one paper in Physical Review Letters, 3 five papers in Science, and one paper in Nature Chemistry since 2001. 4–8 His research has been the subject of two accompanying Science perspective articles. 9,10 The overall relevance to the broader physics and chemistry communities has been underlined by the appearance of general interest reports of Alexander’s work in the professional journals Physics Today, 11 World of Chemistry, 12 and Chemical and Engineering News. 13
1. M. H. Alexander, Nature Chemistry 5, 253 (2013).
2. S. A. Lahankar, J. Zhang, K. G. McKendrick, and T. K. Minton, Nature Chemistry 5, 315–319 (2013).
3. N. Balucani, D. Skouteris, L. Cartechini, G. Capozza, E. Segoloni, P. Casavecchia, M. H. Alexander, G. Capecchi, and H.-J. Werner, Phys. Rev. Lett. 91, 013201 (2003).
4. L. Che, Z. Ren, X. Wang, W. Dong, D. Dai, X. Wang, D. H. Zhang, X. Yang, G. Li, H.-J. Werner, F. Lique, and M. H. Alexander, Science 317, 1061 (2007).
5. H. Kohguchi, T. Suzuki, and M. H. Alexander, Science 294, 832 (2001).
6. M. H. Alexander, G. Capecchi, and H.-J. Werner, Science 296, 715 (2002).
7. E. Garand, J. Zhou, D. E. Manolopoulos, M. H. Alexander, and D. M. Neumark, Science 319, 72 (2008).
8. M. H. Alexander, Science 331, 411 (2011).
9. D. E. Manolopoulos, Science 296, 664 (2002).
10. J. M. Bowman, Science 319, 40 (2008).
8. C. Day, Phys. Today 55, 13 (2002).
9. S. Hadlington, Chem. World 5, http://www.rsc.org/chemistryworld/News/2008/January/03010802.asp#.
10. J. Kemsley, Chem. Eng. News 86, 29 (2008).


