
Education
- Ph.D. Materials Science, May 2009, Department of Materials Univ. of California, Santa Barbara, CA
- B.S. Materials Science, June 2003, Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA
Professional Experience
- Professor, Department of Chemistry and Biochemistry, 2022 -- present
- Associate Professor, Department of Chemistry and Biochemistry, 2018 – 2022
- Assistant Professor, Department of Chemistry and Biochemistry, 2012 – 2018
- Affiliate Professor, Department of Materials Science and Engineering, 2013 – present
- Affiliate Professor, Department of Physics, 2015 – present
- National Research Council Post-Doctoral Fellow, NIST Center for Neutron Research, National Institute of Standards and Technology, 2009-2012
- Graduate Research Assistant and Post-baccalaureate Researcher, Los Alamos National Laboratories, Los Alamos, NM, 2003-2009
- Board of Directors of the American Institute of Physics
- UMD Quantum Materials Center
- UMD Chemical Physics Program
- Graduate Program Director for Chemistry (2018-2021)
- Co-chair of the science program of the 2018 American Conference on Neutron Scattering
- Tobin J. Marks Lecture on Chemical Discovery, chair (2020)
- Co-organizer of annual Fundamentals of Quantum Materials Winter School
- Memberships in: American Chemical Society, American Physical Society, Neutron Scattering Society of America, American Crystallographic Association, and the Society for the Advancement of Chicanos and Native Americans in Science
Research Interests
Materials and solid state chemistry. Inorganic quantum materials. Synthesis and characterization of transition metal oxides and chalcogenides. Neutron scattering investigation of magnetic materials. Exploratory synthesis of quantum materials using solvothermal and hydrothermal methods. Preparation of mesoporous metal oxides for defeat of chemical warfare agents. Structure-property relationships through crystallographic studies.
Major Recognitions and Honors
- Alexander von Humboldt Fellowship for Experienced Researchers
- Margaret C. Etter Early Career Award in Crystallography
- NSF CAREER Award
- Office of Naval Research, MURI on Superconductivity
- DOE Basic Energy Sciences grant in neutron scattering
- UMD Graduate School Research and Scholarship Award
- National Research Council Postdoctoral Fellow at NIST
Significant Professional Service and Activities
- Faculty Co-Advisor of the American Chemical Society Club and Dept. of Chemistry and Biochemistry’s Graduate Student Organization
- Co-organizer of the first Neutron Day at the University of Maryland
- Co-chair of the science program of the 2018 American Conference on Neutron Scattering
- Co-organizer of symposia: ACS National Meeting Synthetic Chemistry Approaches to Magnetic Materials and Emergent Phenomena in the Solid State; American Crystallographic Association Meeting Materials Discovery and Crystal Growth
- Co-organizer of the schools Fundamentals of Quantum Materials and School on Representational Analysis and Magnetic Structures
- Memberships in American Chemical Society, American Physical Society, Neutron Scattering Society of America, American Crystallographic Association, Society for the Advancement of Chicanos and Native Americans in Science
- Other Campus Affiliations: Center for Nanophysics and Advanced Materials, UMD NanoCenter, UMD Energy Research Center, Chemical Physics Program
- Outreach: Johns Hopkins Center for Talented Youth, Science and Technology Series for Spring 2017 & 2015; Panelist and participant for bilingual event titled Sábado de Ciencas and Paving the Way: Event for Latino High School Parents and Students, Northwestern High School, Fall 2014; Co-organizer and panelist Workshop STEM Expo for Parents sponsored by the Office for Minorities in Science and Engineering, 2014.
Students Mentored
- Advised 7 PhD students, who now work in diverse areas including Ford Motor Company, Intel Corporation, Naval Academy, Northwestern University, and the National Institute of Standards and Technology
- Currently lead a team of 7 graduate students and 2 research postdoctoral associates
- Mentored 14 undergraduate interns in the laboratory
- 4 undergraduate interns were Louis Stokes Alliance for Minority Participation (LSAMP) Fellows
Solid state materials mediate how we utilize energy, communicate, and store information. Through chemistry we can control the physical properties of solids and solve some of society’s grand challenges in energy, computing, and human health. Our solid state chemistry laboratory tackles these grand challenges through a multidisciplinary approach. Therefore, our research combines chemical synthesis with advanced characterization of physical properties and atomic structures.
New superconductors and layered transition metal chalcogenides
 Our group leads in the preparation of new superconductors, which are materials that conduct an electrical current without any resistive losses below a certain critical temperature (Tc). We have focused our studies on iron-based superconductors, and specifically layered iron chalcogenides where a chalcogen is a group 16 element of the periodic table such as sulfur, selenium, or tellurium.[1-3] The figure below shows the key structural motif of these quantum materials, which consists of a two dimensional layer composed of a square lattice where each iron center is sandwiched between two layers of either sulfide or selenide anions.
Our group leads in the preparation of new superconductors, which are materials that conduct an electrical current without any resistive losses below a certain critical temperature (Tc). We have focused our studies on iron-based superconductors, and specifically layered iron chalcogenides where a chalcogen is a group 16 element of the periodic table such as sulfur, selenium, or tellurium.[1-3] The figure below shows the key structural motif of these quantum materials, which consists of a two dimensional layer composed of a square lattice where each iron center is sandwiched between two layers of either sulfide or selenide anions.
Transition metal oxides with exotic magnetic properties
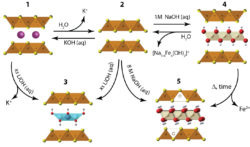
 Magnetic materials can be utilized in applications such as magnetic memory storage, and exhibit exotic ground state properties. We use our materials chemistry approach to explore new physics in magnetic materials. We focus on transition metal oxides, and especially those with so-called hollandite-type structure to explore this field further. [4] The main structural motif consists of a triangular ladder that connect the transition metal centers, and these hollandites are an interesting platform to study the interplay of charge, spin, and orbital degrees of freedom. In addition, we are also currently exploring a new area of magnetism known as ferrotorodic materials. Our goal is to prepare and study the magneto-structural properties of what have been considered the missing fourth class of primary ferroics–ferrotoroidics.
Magnetic materials can be utilized in applications such as magnetic memory storage, and exhibit exotic ground state properties. We use our materials chemistry approach to explore new physics in magnetic materials. We focus on transition metal oxides, and especially those with so-called hollandite-type structure to explore this field further. [4] The main structural motif consists of a triangular ladder that connect the transition metal centers, and these hollandites are an interesting platform to study the interplay of charge, spin, and orbital degrees of freedom. In addition, we are also currently exploring a new area of magnetism known as ferrotorodic materials. Our goal is to prepare and study the magneto-structural properties of what have been considered the missing fourth class of primary ferroics–ferrotoroidics.
Transition metal oxides for energy conversion
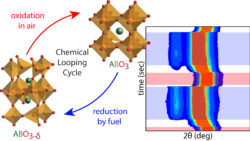
We also study energy conversion materials. An alternative method to convert fuels into energy besides combustion with air is to react them with solid metal oxides at elevated temperatures (> 600 °C). Since these solids are depleted of their oxygen content after reaction with a fuel, they will have to be regenerated in air in order to restore the amount of oxygen in the crystal lattice. Hence, these materials are ‘oxygen sponges’ known as oxygen storage materials (OSMs). As illustrated below these OSMs are the key participants in what is known as a Chemical Looping Cycle (CLC). Successful and widespread use of CLC reactors would cut down on the emissions of the most damaging greenhouse gas, CO2, and it would preclude expensive gas separations. In our laboratory we synthesize OSMs by focusing on transition metal oxides study their performance under CLC conditions while monitoring their chemical composition and crystal structures.[5,6]
References
- Zhou, X.; Eckberg, C.; Wilfong, B.; Liou, S.-C.; Vivanco, H. K.; Paglione, J.; Rodriguez, E. E.*, “Superconductivity and magnetism in iron sulfides intercalated by metal hydroxides”, Chemical Sciences, 2017, 8, 3781-3788.
- Zhou, X.; Rodriguez, E. E.*, “Tetrahedral transition metal chalcogenides as functional inorganic materials”, Chemistry of Materials, 2017, available online.
- Zhou, X.; Wilfong, B.; Vivanco, H. K.; Paglione, J.; Brown, C. M.; Rodriguez, E. E.*, “Layered metastable cobalt chalcogenides from topochemical deintercalation”, Journal of the American Chemical Society, 2016, 138, 16432.
- Larson, A.; Wilfong, B.; Moetakef, P.; Brown, C. M.; Zavalij, P.; Rodriguez, E. E.*, “Metal–insulator transition tuned by magnetic field in Bi1.7V8O16 hollandite”, Journal of Materials Chemistry C, 2017, 5, 4967-4976.
- Taylor, Daniel D.; Schreiber, N.; Levitas, B.; Xu, W.; Whitfield, P.; Rodriguez, E. E.*, “Oxygen storage properties of La1−xSrxFeO3−δ for chemical-looping reactions-an in-situ neutron and synchrotron X-ray study”, Chemistry of Materials, 2016, 28, 3951-3960.
- Liu, L.; Taylor, D. D.; Rodriguez, E. E.; Zachariah, M. R., “Influence of transition metal electronegativity on the oxygen storage capacity of perovskite oxides”, Chemical Communications, 2016, 52, 10369-10372.


